- 高純度化学研究所
- ENGLISH
- Product Guide
- Metals and Inorganic materials
- Functional Materials
Functional Materials
What's Functional Materials?
We have been producing various functional materials, using our own technologies. Representative ones of them include ”Constant temperature holding material Smartec®-HS” and “Thermal expansion suppressor Smartec®” and “Lanthagen ®”.
Functional material
Constant temperature holding material (Heat storage material)
〈product name: Smartec® HS〉
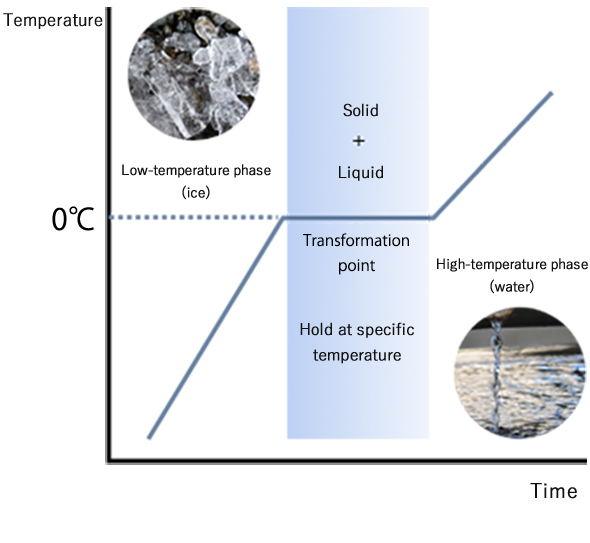
A constant temperature holding material is a material using “heat storage characteristic” of some substance. “Heat storage material” is a general name for materials for holding temperature constant by the use of a large heat capacity of the substance. A representative one is that which uses heat of transition between ice and water.
In 2011, it was discovered by the National Research and Development Agency, Institute of Physical and Chemical Research (RIKEN) that a substance obtained by substituting part of vanadium dioxide (VO2) with another metal has large heat storage cap ability, and RIKEN has acquired a patent of a heat storage material of a type which enables optional selection of holding temperature.
We started working on the material very soon, and have licensed first the patent and related rights from RIKEN.
Previously developed materials use release and absorption of heat during transition between solid and liquid phases, and accordingly have problems in such as that: there arises restriction on the container because of liquefying and large volume change; they show low thermal response due to low thermal conductivity; they themselves decompose; holding temperature is limited to the transition temperature specific to the substance. In contrast to that almost all other heat storage materials use solid-liquid phase transition, the present material uses large latent heat of VO2 and has the most significant feature of using solid-solid phase transition.

conventional heat storage material(water/ ice)
vanadium dioxide based heat storage material
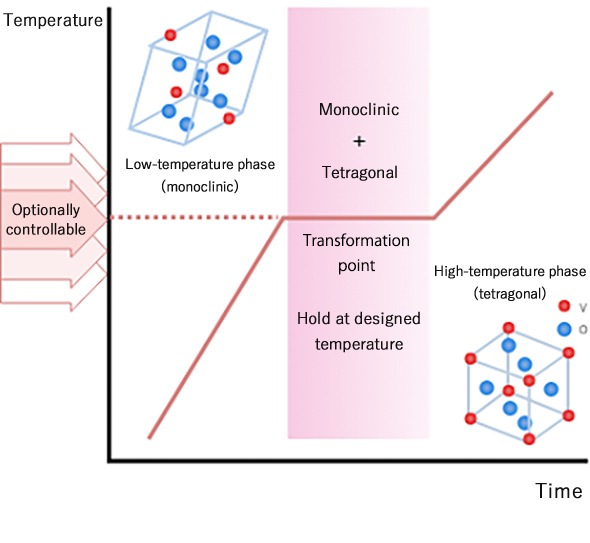
For example, among the series of newly developed materials, V0.977W0.023O2 having heat storage ability at 11℃ shows performance of temperature holding at 11℃ under room temperature environment, which is almost equivalent to ice’s performance of temperature holding at 0℃ under the same environment. The holding temperature is not limited to around room temperature but can be controlled to vary from a low temperature (-30℃) to near a high temperature (200℃) by varying an element to substitute for vanadium and the substitution rate.
Standard line-up of heat storage materials
| “Smartec® HS 10” for low temperature region, control temperature: around 10 deg. C. |
| “Smartec® HS 70” for high temperature region, control temperature: around 70 deg. C. |
| “Smartec® HS 120” for high temperature region, control temperature: around 120 deg. C. |
※About controlling temperature different from the above ones, please consult with us any time.
Merits of the present Constant Temperature Holding Material
in comparison to conventional heat storage materials,
- It is in a solid form, and requires no restriction on the container
- It can be processed into various shapes by mixing with resin and so on
- The holding temperature can be optionally designed by composition control
- It has high thermal conductivity, and accordingly has high thermal responsibility
- It causes no excessive cooling and, differently from specific-heat type materials, hold a constant temperature
- It is non-combustible and has a high heatproof temperature
The present constant temperature holding material is expected to be applied to uses where abrupt temperature variation is not acceptable, such as structural and transportable materials for electronic materials and equipment.
Thermal Expansion Suppressor
<product name Smartec®>
This material, discovered at RIKEN in 2005, has a negative thermal expansion coefficient in a certain temperature range. We have worked on commercialization of the material, adopting our nitride synthesis technology, and have licensed the invention from RIKEN. In the commercialization effort, we have adopted sintering technologies we built up through manufacturing of functional ceramics powders, sputtering targets, EB evaporation materials and so on.
A temperature range where this material effectively works can be adjusted by varying the constituent elements and the composition.Please consult with us.
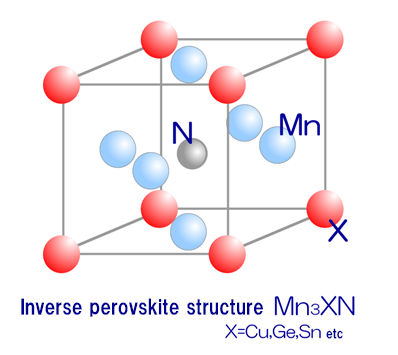
Feature of manganese nitrides
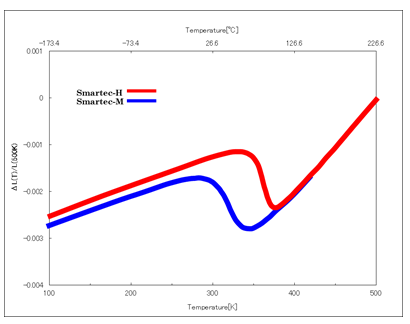
It was already known that manganese nitrides having an inverse perovskite structure, such as Mn3ZnN, show abrupt volume reduction due to phase change with increasing temperature. By partially substituting these manganese nitrides with germanium, tin or the like, the volume reduction due to phase change can be relaxed. Sintered products of such materials show a negative thermal expansion coefficient continuously over a certain temperature range, as shown in the below figure. The materials are expected to be applied in the fields of precision equipment, temperature compensated materials and so on, and accordingly have attracted attention.
Examples of our achievements regarding the thermal expansion suppressor
※You can scroll and see
| products for normal temperature use | control range: 20 to 65℃, thermal expansion coefficient α= -40 ppm/℃ |
|---|---|
| products for high temperature use | control range: 65 to 100℃, thermal expansion coefficient α= -45 ppm/℃ |
| Other | provision of information about a material for injection-molding plastics, in the form of resin-mixing pellets |
Merits of using the manganese nitrides
in comparison to conventional heat storage materials,
- large negative thermal expansion coefficient
- degree of negative thermal expansion is easy to control
- no anisotropy in negative thermal expansion, can be used in powder form
- injection-molding plastic process is enabled by mixing with resin
- eco-friendly material

Lanthagen®
We have conducted research and development, production and sales on various kinds of metal alkoxides for many years, and have accumulated experiences and technologies abundantly. Generally, metal alkoxides have been used for sol-gel,CSD and MOCVD methods, which are methods for producing metal oxides in thin film or bulk form. In recent years, alkoxides of lanthanides such as lanthanum have attracted attention as source materials for asymmetric catalysts. In such a situation, our lanthanide alkoxide products have been highly valued, in catalytic activity, by a number of customers.
We have developed a catalytic activity evaluation method for evaluating lanthanide alkoxides for asymmetric catalysts, under the guidance of Prof. Masakatsu Shibasaki at Graduate School of Pharmaceutical Sciences, The University of Tokyo. We have determined that each and every production lot is evaluated using the evaluation method and only acceptable products are provided under the name of "Lanthagen®”.
Here, we would like to introduce Lanthagen® , which would be helpful to you in research and development, and trial or full-scale production.
Outline of the evaluation method
Examples of reaction 1, 2
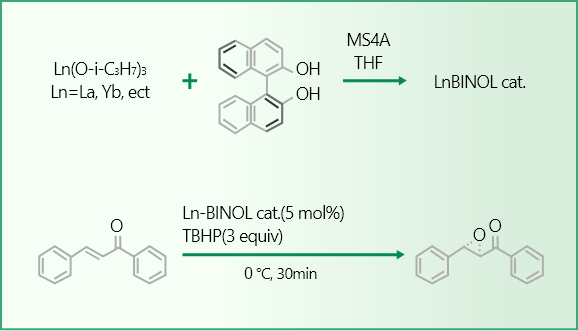
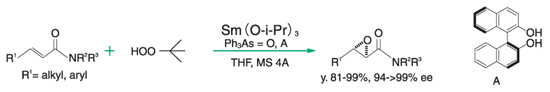
A catalytic activity evaluation value is expressed by the reaction rate and asymmetric yield in asymmetric epoxidation reaction of chalcone which is performed using a Ln-BINOL catalyst prepared from a lanthanide alkoxide and BINOL ((S)-2,2′-dihydroxy-1,1′-binaphthyl).
The reaction in the evaluation method is performed under a condition of lower temperature, shorter reaction time and no additives such as Ph3As=O, so as to ease identifying the catalytic performance. Therefore, thus measured values of the reaction rate and asymmetric yield both are lower, compared to data on asymmetric epoxidation reaction of chalcone described in literature and the like.
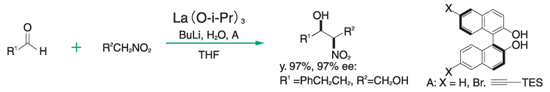
Nitro-aldol reaction³
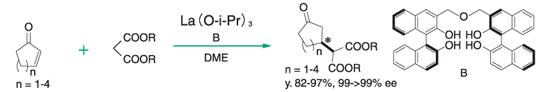
Michael reaction⁴
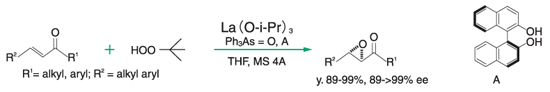
Epoxidation reaction of enone⁵,⁶
Epoxidation reaction of α.β-unsaturated N-acylimidazole⁷
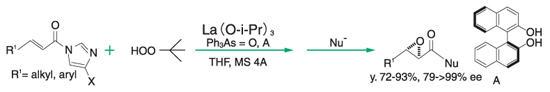
Epoxidation reaction of α.β-unsaturated amide⁸
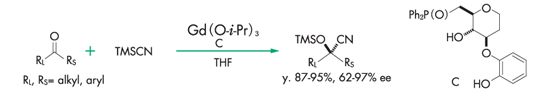
Cyanosilylation reaction of ketone⁹,10
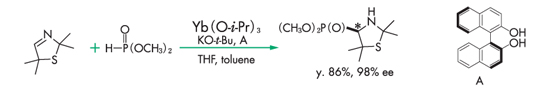
Hydrophosphonylation reaction11
The above data is shown by courtesy of Prof. Masakatsu Sibasaki, The university of Tokyo.
Line-up of lanthanide alokoxides for catalysts
※You can scroll and see
| Code No. | LAR04GB | PRR02GB | SMR02GB | GDR02GB | YBR02GB | YYR04GB | |
|---|---|---|---|---|---|---|---|
| Product name | Lanthagen® Tri-isopropoxy lanthanum |
Lanthagen® Tri-isopropoxy praseodymium |
Lanthagen® Tri-isopropoxy samarium |
Lanthagen® Tri-isopropoxy gadolinium |
Lanthagen® Tri-isopropoxy ytterbium |
Lanthagen® Tri-isopropoxy yttrium |
|
| Chemical formula | La(O-i-C3H7)3 | Pr(O-i-C3H7)3 | Sm(O-i-C3H7)3 | Gd(O-i-C3H7)3 | Yb(O-i-C3H7)3 | Y(O-i-C3H7)3 | |
| General name | Lanthanum tri-isopropoxide |
Praseodymium tri-isopropoxide |
Samarium tri-isopropoxide |
Gadolinium tri-isopropoxide |
Ytterbium tri-isopropoxide |
Yttrium tri-isopropoxide |
|
| CAS No. | 19446-52-7 | 19236-14-7 | 3504-40-3 | 14532-05-9 | 6742-69-4 | 2172-12-5 | |
| Molecular weight | 316.2 | 318.2 | 327.6 | 334.5 | 350.3 | 266.2 | |
| Appearance | White solid | Light green solid | Yellow-orange solid | White solid | White solid | White solid | |
| Degree of association*1 | - | - | - | - | - | 4.0 | |
| Solubility (g/L)*2 | THF | 320 | 300 | 470 | 250 | 310 | 240 |
| Toluene | 200 | 220 | 280 | 290 | 270 | 200 | |
| Hexane | 270 | 310 | 470 | 250 | 310 | 240 | |
| IPA | 80 | 50 | 40 | 30 | 180 | 20 | |
| Content of rare earth metals*3 (%) |
43.9 | 46.0 | 48.3 | 50.0 | 47.6 | 37.3 | |
| Impurities*3 (%) |
Al | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 |
| Ca | <0.01 | 0.06 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Fe | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Si | <0.01 | <0.01 | 0.03 | 0.03 | <0.02 | 0.03 | |
| Na | 0.30 | 0.10 | 0.13 | 0.52 | <0.01 | <0.01 | |
| Cl | 0.68 | 1.0 | 0.47 | 0.26 | 0.02 | 0.06 | |
| Catalytic performance | yield(%) | ≥55 | ≥63 | ≥54 | ≥82 | ≥41 | ≥84 |
| ee(%ee) | ≥70 | ≥51 | ≥56 | ≥51 | ≥62 | ≥15 | |
*1 The degree of association was calculated from a molecular weight value measured by the benzene cryoscopic method.
*2 The solubility is expressed by the amount of solute in one liter of solution at room temperature.
*3 For the contents of rare earth metals and of impurities, typical measured values are shown. “-” indicates that no measured value is available.
Handling Precautions
- The products are contained in a glass ampoule filled with an inert gas.
- The lanthanide alkoxides decompose if contacting with even a slight amount of moisture in the air, and thereby lose catalytic activity. Please handle the products in a glove box or a glove bag sufficiently filled with a dry inert gas.
- Once opening an ampoule, please use it up immediately. Even if stored in a glass bottle or a glove box, there occurs reaction with a slight amount of moisture and resultant reduction in catalytic activity.
- Please dehydrate a solvent sufficiently, before using it to dissolve the products.
